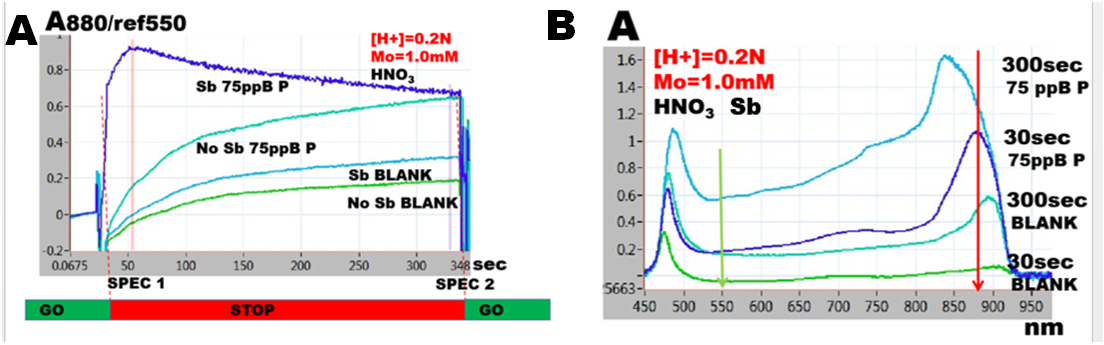
While the objective of this study, optimization of PMoB method, to be used for trace analysis of phosphate was achieved, many unknowns about PMoB chemistry remain hidden in the black box. While this study implies a simple situation, interaction between reaction rates of formation of PMoB and of reagent blank (MoB), the literature offers far more complex picture, where various PMoB species are formed in presence of various reductants and with and without Sb intervention. (Nagul et.al. 2015 Fig.6). Whether the differences of values of molar absorbtivities reported in literature are caused by measuring a blend of colloidal suspension (Hatta et.al, 2019) produced at suboptimal conditions, or due to formation of different PMoB compounds still has to be resolved.
Hopefully, further studies of fascinating chemistry of phospho and silica molybdates will continue, with assistance of programmable Flow Injection technique and associated instrumentation, that made this initial work possible.